what are saturated hydrocarbons give example Ncert class x science class: chapter –4. carbon and its compounds
Hydrocarbons are a type of organic compound that is composed of carbon and hydrogen atoms. These compounds are found in many different forms and are used in a wide variety of applications. From simple chains to complex ring structures, hydrocarbons are a crucial part of our daily lives. In this post, we will discuss the different types of hydrocarbons and their uses.
Saturated Hydrocarbons
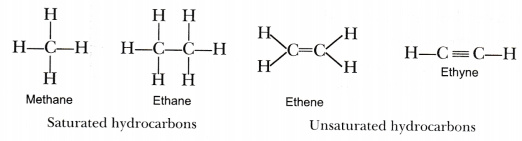
Saturated hydrocarbons, also known as alkanes, are hydrocarbons that only contain single covalent bonds between carbon atoms. They have a general formula of CnH2n+2 and are highly stable and unreactive. Some of the most common saturated hydrocarbons are methane, propane, and butane. These hydrocarbons are commonly used as fuel sources, especially propane and butane, which are commonly used in cooking and heating.
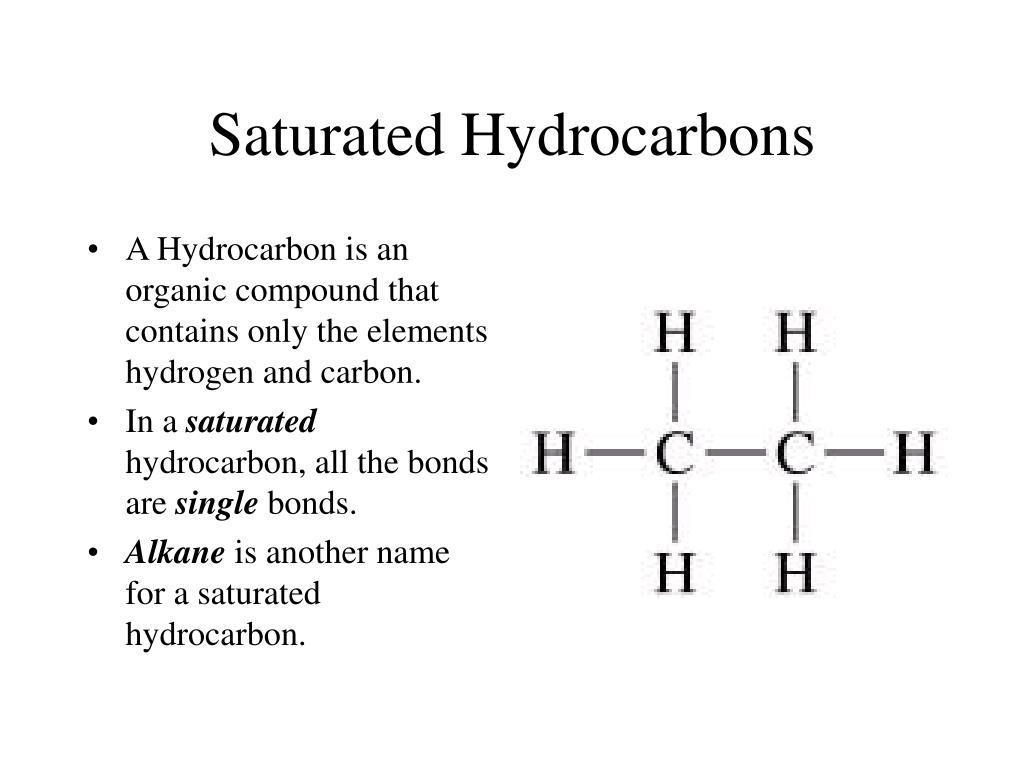 In addition to their use as fuel sources, saturated hydrocarbons are also used in the production of plastics, synthetic fibers, and lubricating oils. The stability of these compounds makes them ideal for use in these applications.
In addition to their use as fuel sources, saturated hydrocarbons are also used in the production of plastics, synthetic fibers, and lubricating oils. The stability of these compounds makes them ideal for use in these applications.
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are hydrocarbons that contain at least one double or triple bond between carbon atoms. They are generally more reactive than saturated hydrocarbons and can be used in a wide variety of applications. Some of the most common unsaturated hydrocarbons are ethene, propene, and butene.
 Unsaturated hydrocarbons are commonly used in the production of plastics, rubber, and other synthetic materials. They are also used as solvents in a variety of applications, including paint thinners and cleaning agents.
Unsaturated hydrocarbons are commonly used in the production of plastics, rubber, and other synthetic materials. They are also used as solvents in a variety of applications, including paint thinners and cleaning agents.
Aromatic Hydrocarbons
Aromatic hydrocarbons, also known as arenes, are hydrocarbons that contain one or more benzene rings. These compounds are highly stable and are used in a variety of applications. Some of the most common aromatic hydrocarbons are benzene, toluene, and xylene.
/aromaticcompounds-58bdb26f5f9b5860461b126f.jpg) Aromatic hydrocarbons are commonly used in the production of chemicals, including dyes, plastics, and detergents. They are also used as solvents in a variety of applications, including paints, coatings, and cleaning agents.
Aromatic hydrocarbons are commonly used in the production of chemicals, including dyes, plastics, and detergents. They are also used as solvents in a variety of applications, including paints, coatings, and cleaning agents.
Hydrocarbons are a crucial part of many different industries, from energy production to manufacturing. Understanding the different types of hydrocarbons and their applications is essential for anyone working in these industries.
If you are looking for Saturated and Un-saturated Hydrocarbons Chemistry you’ve came to the right place. We have 5 Pics about Saturated and Un-saturated Hydrocarbons Chemistry like NCERT Class X Science Class: Chapter –4. Carbon and Its Compounds, Saturated and Un-saturated Hydrocarbons Chemistry and also What are hydrocarbons? Give examples - CBSE Class 10 Science - Learn. Here you go:
Saturated And Un-saturated Hydrocarbons Chemistry
 www.knowledgeuniverseonline.comhydrocarbons saturated unsaturated carbon chemistry un
www.knowledgeuniverseonline.comhydrocarbons saturated unsaturated carbon chemistry un
What Are Hydrocarbons? Give Examples - CBSE Class 10 Science - Learn
 ask.learncbse.inhydrocarbons examples carbon unsaturated give bond least triple double class group contain atoms atom
ask.learncbse.inhydrocarbons examples carbon unsaturated give bond least triple double class group contain atoms atom
NCERT Class X Science Class: Chapter –4. Carbon And Its Compounds
 www.flexiprep.comcarbon compounds unsaturated hydrocarbons class saturated bond triple double ncert science its part least structural differences flexiprep chapter contain
www.flexiprep.comcarbon compounds unsaturated hydrocarbons class saturated bond triple double ncert science its part least structural differences flexiprep chapter contain
Unsaturated Hydrocarbons:
 www.nextgurukul.inPPT - Carbon Compounds PowerPoint Presentation, Free Download - ID:174669
www.nextgurukul.inPPT - Carbon Compounds PowerPoint Presentation, Free Download - ID:174669
 www.slideserve.comsaturated hydrocarbons carbon compounds hydrocarbon compound ppt powerpoint presentation alkane name hydrogen slideserve properties
www.slideserve.comsaturated hydrocarbons carbon compounds hydrocarbon compound ppt powerpoint presentation alkane name hydrogen slideserve properties
Unsaturated hydrocarbons:. Ncert class x science class: chapter –4. carbon and its compounds. What are hydrocarbons? give examples